Data Acquisition and Analysis Services
Welcome to mores.info, your trusted partner for DAAS solutions. Our services cater to diverse industries, ensuring precision and compliance. Data Acquisition and Analysis System as it might be a proprietary or specialized system developed by mores.info
What We Do?
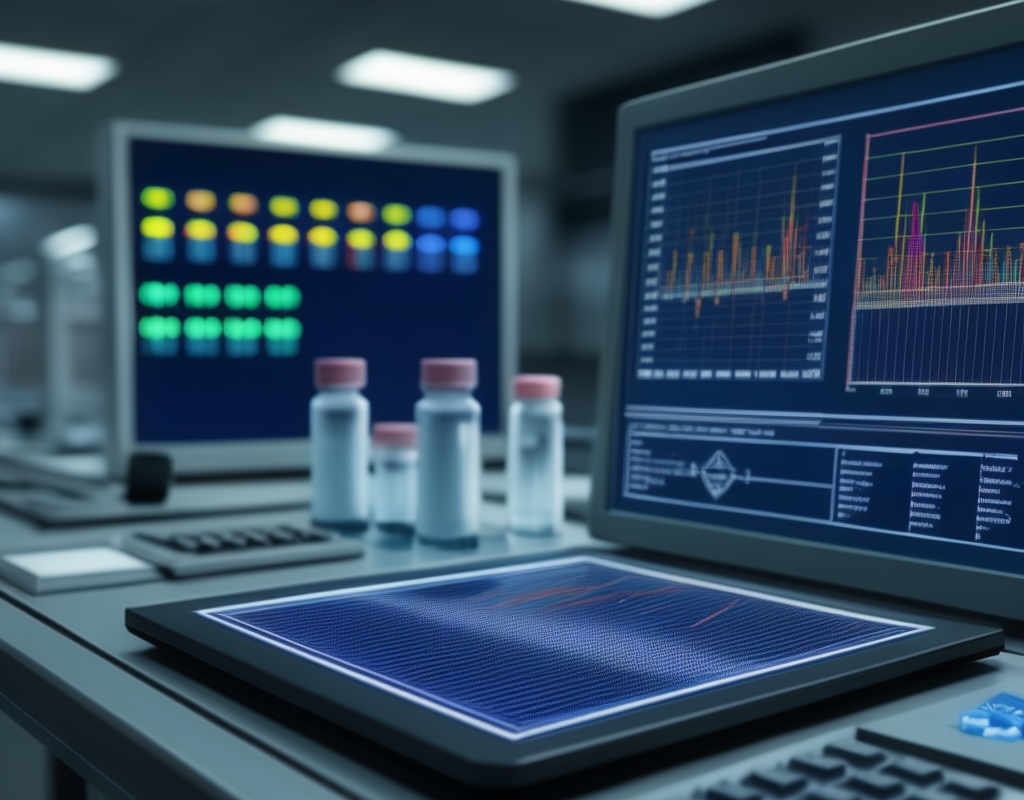
Data Acquisition and Analysis System, Make Project for pharmaceutical sector, in which we are communicating with different PLC (Allen-Bradley, Siemens, B&R, Delta, Mitsubishi, OMRON, etc.) and acquiring data for analysis and audit report. This project follows the FDA rule 21 CFR Part 11 compliance.
Data Acquisition
Data Acquisition (DAQ) where we collecting and measuring information from various sources such as PLC, Data Logger, Controller, Sensors, Instruments, and IoT or Convertor (Serial to LAN) devices. It can be used for monitoring, control, and decision-making in various fields like pharmaceutical, automobile, healthcare.
Analysis System
An analysis system refers to the tools, software, and methodologies used to interpret and make sense of collected data. The goal of analysis systems is to extract meaningful insights, patterns, and knowledge from raw data, enabling informed decision-making.
Data Acquisition and Analysis
Data Acquisition and Analysis suggests that this is the fourth iteration or release of the data acquisition and analysis system. Version updates often include improvements, new features, and bug fixes based on user feedback and evolving requirements.
Key Features and Components
PLC Communication
The system is equipped to establish communication with PLCs from diverse manufacturers, ensuring compatibility with systems commonly used in the pharmaceutical sector
Data Acquisition
It efficiently collects and retrieves data from the PLCs, capturing real-time information related to various processes within the pharmaceutical manufacturing environment.
Analysis Capabilities
The system incorporates advanced analytical tools to process the acquired data. This may involve statistical analysis, trend identification, and other methodologies to derive meaningful insights from the data collected.
Audit Trail Generation
A crucial aspect of the system is its ability to create detailed audit trails. This is vital for compliance with FDA regulations (21 CFR Part 11) which require electronic records to be secure, reliable, and traceable.
Security Measures
Given the regulatory context, the system likely implements robust security measures to ensure the integrity and confidentiality of the data. This includes user authentication, access controls, and data encryption.
User Interface
The system is expected to have a user-friendly interface that allows pharmaceutical professionals to interact with and manage the acquired data. This interface might include dashboards, visualization tools, and reporting features.
Regulatory Compliance
Compliance with FDA rule 21 CFR Part 11 is a fundamental aspect of the system. This regulation specifically addresses electronic records and electronic signatures, ensuring that they are trustworthy, reliable, and equivalent to paper records.
Version 4.0
Version 4.0 designation implies that the system has undergone iterative improvements. These enhancements could involve optimizations in performance, additional features, bug fixes, and other upgrades to make the system more effective and user-friendly.
Smart Solutions, Smart Data: Elevate Your Operations with mores.info
Modules That We Work
Types of Data Acquisition and Analysis Systems
Pharmaceutical Data Acquisition Systems
Pharmaceutical sector acquire data from programmable logic controllers (PLCs) for analysis and compliance reporting, following regulations such as FDA rule 21 CFR Part 11.
Automotive Data Acquisition Systems
Automotive industry, these systems collect data from sensors and control units in vehicles. This data is used for performance analysis, diagnostics, and Pick to Light, Poke Yoke System.
Structural Health Monitoring Systems
Used to monitor the health and integrity of structures such as bridges, buildings, and dams. These systems collect data from sensors to detect and assess structural issues.
Industry We Serves

Pharma
Automobile

Sugar Factory
Casting Industry

